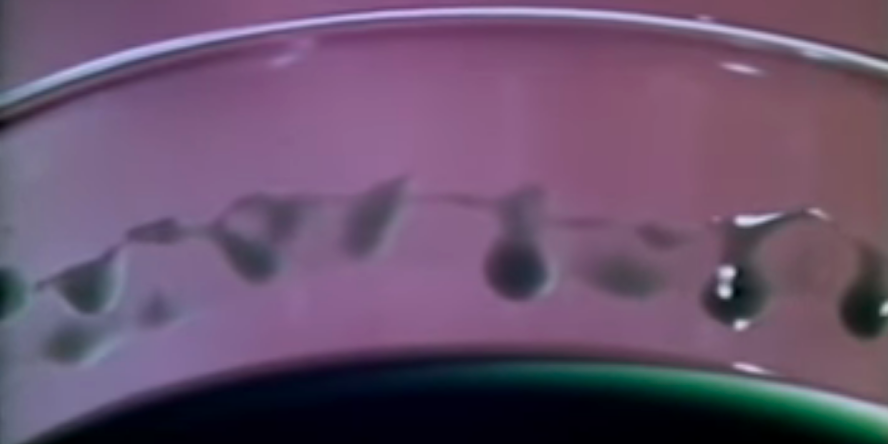Course Video: Surface Tension in Fluid Mechanics
9.14.18
The man in this video starts to demonstrate various surface tension concepts in an easy to learn, comprehensive way. By the 3 minute mark I've already understood that these systems involving surface tension go towards a conformation of minimal energy within the system. When he places a drop of mercury onto a plane of glass he defines that if the angle with which the liquid meets a sold we say that the "liquid does NOT wet the solid". This is interesting and I think it comes from the molecules of a fluid wanting to interact within the fluid than the solid it's trying to adhere to. This then makes sense to me.
I've learned that if we keep with the regime that surface exert forces then we have the following boundary conditions as well.
- Where surfaces meet, the contact angle is uniquely determined by the energies of the interfaces.
- A curved liquid surface has a higher pressure on the concave side.
- If the surface tension varies along the surface then the surface must move. (Variation of surface tension along a surface is balanced by shear forces in the bounding materials).
He then defines the pressure difference within a spherical bubble to the surrounding volume as Δ P = (2 * σ) / R, where R is the radius of the bubble.
Then moving on he let's us know that the smaller a bubble the more its radius shrinks the pressure difference required becomes infinite. So in fact creating new bubbles out of nowhere, like in a pot of boiling water, is very rare. Most bubble that we observe in that pot are actually the cause of tiny cracks or other imperfections serving as nucleation sites.
Showing a facuet with a small cylinder of water he demonstrates how it will always split into a set of drops. And then shows how this process happens in slow motions. The cylinder pinches off as pressure varies along the length of the cylinder. The water flowing onto the top of a cylinder reminds me of a paper I read on how chocolate fountains work which you can find here.
I don't necessarily understand the wine tears portion of this video, but the concentration gradient between the drops and bulk fluid could lead to the difficulty in mixing.
Overall this video was a very good introduction to fluid dynamics and I look forward to this class for the rest of the semester!