High aerosol acidity despite declining atmospheric sulfate conecentrations over the past 15 years
4.27.19
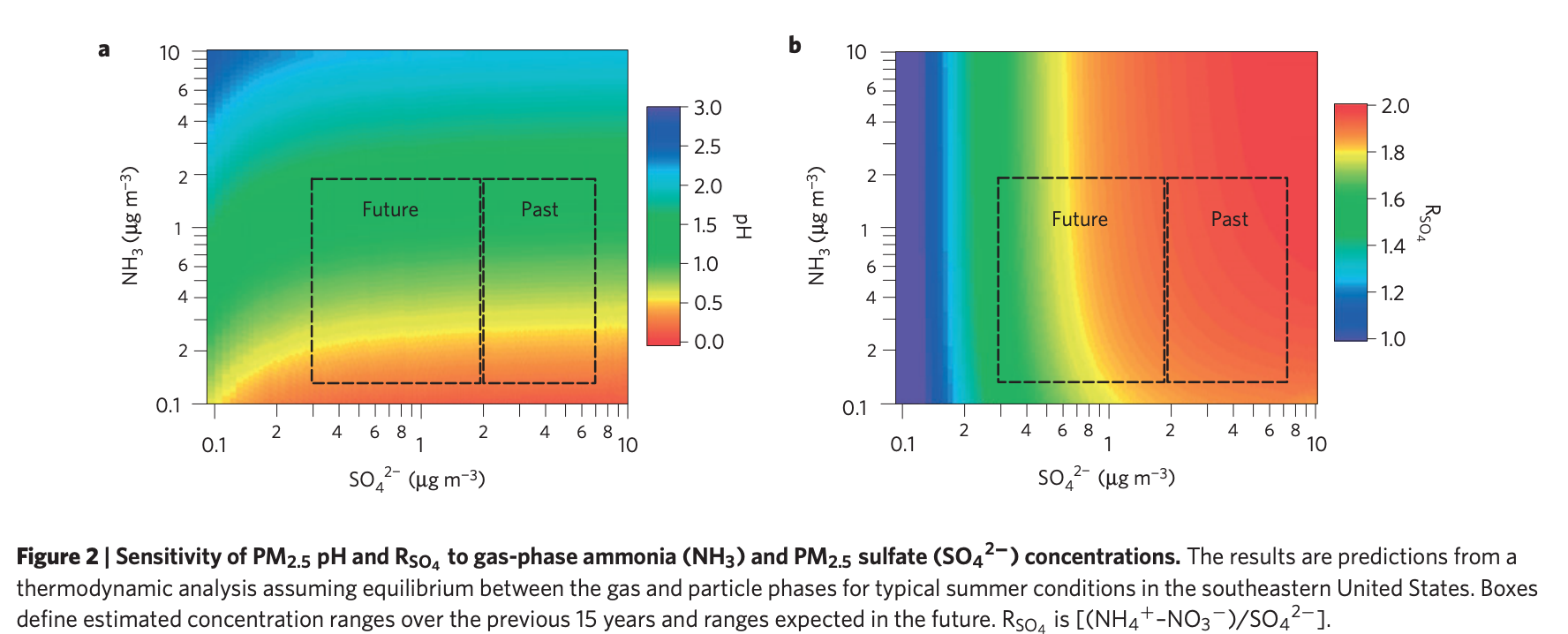
When we think about acid rain we're not thinking about the complex reactions that lead to an increase in the overall pH within the atmosphere. Acidic PM2.5 particles reach their low pH values mainly due to sulfate concentrations and this was a huge problem about 20 years ago. This paper sheds light on how even though sulfate concentrations have declined by 70%
over the past 15 years the aerosols are still highly acidic. We've decreased atmospheric sulfate concentrations through policies that enforce coal plants to install scrubbers and other filter methods. But a buffer caused by NH3 in the atmosphere partitioning into aerosol particles has kept the pH values so low. They explain that NH3 concentrations haven't been touched by policy yet, and are mainly released in connection with agricultural activities
. When pH levels are high it's connected to a variety of different effects such as higher PM2.5 formation, higher metal solubility, and direct respiratory effects
.
When they did this study they used samples that weren't from any time period when there were rain or dust storms. They also used data from the same areas and noted that areas with higher NH3 concentrations were in fact agricultural sites. They used ISORROPIA-II as their thermodynamic model to make aerosol pH predictions. This model predicts composition and phase of various ions in aerosols as balanced by their gas phase precursors
. This model is coded primarily in Fortran 77, a language I'm completely unfamiliar with. With some digging I'm realizing that Fortran is first of all, still widely used, even in the West research group where it's used for simulations of our electrochemical systems. It's used extensively for numerical and scientific computing and even though it's not a language that I've used in my data analytics projects it's utilized anywhere where the problems are computationally intensive. I've seen it mentioned now in total in electrochemistry, computational chemistry, atmospheric chemistry, and computational fluid dynamic work.
The model does a fantastic job of predicting the NH3 compositions from mid to late June of 2013, demonstrating the rigor of the computations. I think that this paper does a good job highlighting that for many phenomena within the atmosphere there isn't just a direct cause and effect because any change will have a sizable ripple effect. It also comes with sobering evidence that unless we cut down on sulfate emissions to pre-anthropogenic levels the pH of the atmosphere will continue to remain this low, acidic value. Once again we have to be willing to cut back on our own greed if we're to take care of this planet in the way that we're obligated to.